As COVID-19 continues to wreak havoc, many scientists and researchers across the world are doing their best to find solutions to lessen the effects of the disease. As of today, there are no specific therapeutic drugs or vaccines to treat COVID-19. Scientists/researchers are trying various methods to treat the patients. Several therapies such as antimalarial drugs (Chloroquine/Hydroxychloroquine), antiviral drugs (Remdesivir), antiparasitic agents (Ivermectin), etc., are being used, but a COVID specific drug is not yet known.
One such treatment is the use of convalescent plasma therapy. The use of convalescent plasma therapy dates back to the 1800s, where it was first used to treat diphtheria. Later, it was used to treat the Spanish flu, measles, influenza, chickenpox, H1N1 and H1N5 avian flu, ebola, etc.
In a study involving influenza patients, the treatment of severe infection with convalescent plasma was associated with reduced respiratory tract viral load, lower mortality, and reduction in the viral infection/symptoms. The rate of hospital discharge was higher in patients who were administered plasma therapy when compared with that of patients who did not receive plasma therapy. These reports raised the hypothesis of using convalescent plasma therapy to treat COVID-19 patients.
After China and the USA, India sought and received the approval of ICMR for convalescent plasma therapy. However, the ICMR does not recommend it as a treatment option outside clinical trials.
What is convalescent plasma therapy?
Convalescent plasma (immune plasma) refers to the plasma that is collected from individuals who have been infected and developed antibodies. Convalescent plasma therapy involves passive antibody therapy, wherein antibodies from the blood of infected people are transfused to those critically affected by the virus. Convalescent plasma therapy is s passive immunization, a preventive measure, and cannot be used for the overall treatment of the disease. The therapy can also be used to immunize those who are at a higher risk of contracting the virus – such as health workers.
Also Read: Cytokine storm syndrome worsens COVID-19 outcomes
How it works
Plasma therapy uses the antibodies developed in individuals who recovered from the infection. The antibodies are produced as an innate response to the infection. They show their therapeutic action through various mechanisms in the body. They bind to the pathogens and kill them, while some other mechanisms involve antibody-mediated cytotoxicity, activation of the complement cascades, and neutralizing the effect of the virus. These antibodies are highly specific and thus eliminate pathogens from the body. When donated to patients, they help fight the infection, possibly reducing the severity of the disease.
Once a person has recovered from the infection, their blood is taken so that the antibodies produced in their body can be collected and used to treat other patients.
Also Read: The role of your GENES in fighting COVID-19
Convalescent plasma collections workflow
Convalescent plasma can be mobilized rapidly using established blood collection and transfusion infrastructure. Standard precautionary measures must be taken while collecting and transfusing the blood.
- Donor eligibility: Firstly, individuals should have a clinical history of the infection. They must be virus free at the time of donating the blood, no sooner than at least 14 days after the resolution of the symptoms.
- Donor recruitment: Blood centers have a well-developed infrastructure for donor recruitment; while they may be best equipped to oversee recruitment in collaboration with partner hospitals, their primary responsibility is to ensure adequate blood supply to meet clinical demands.
- Pre-donation screening: Donors are screened for the virus, or the presence of any other diseases such as hepatitis, HIV, etc.
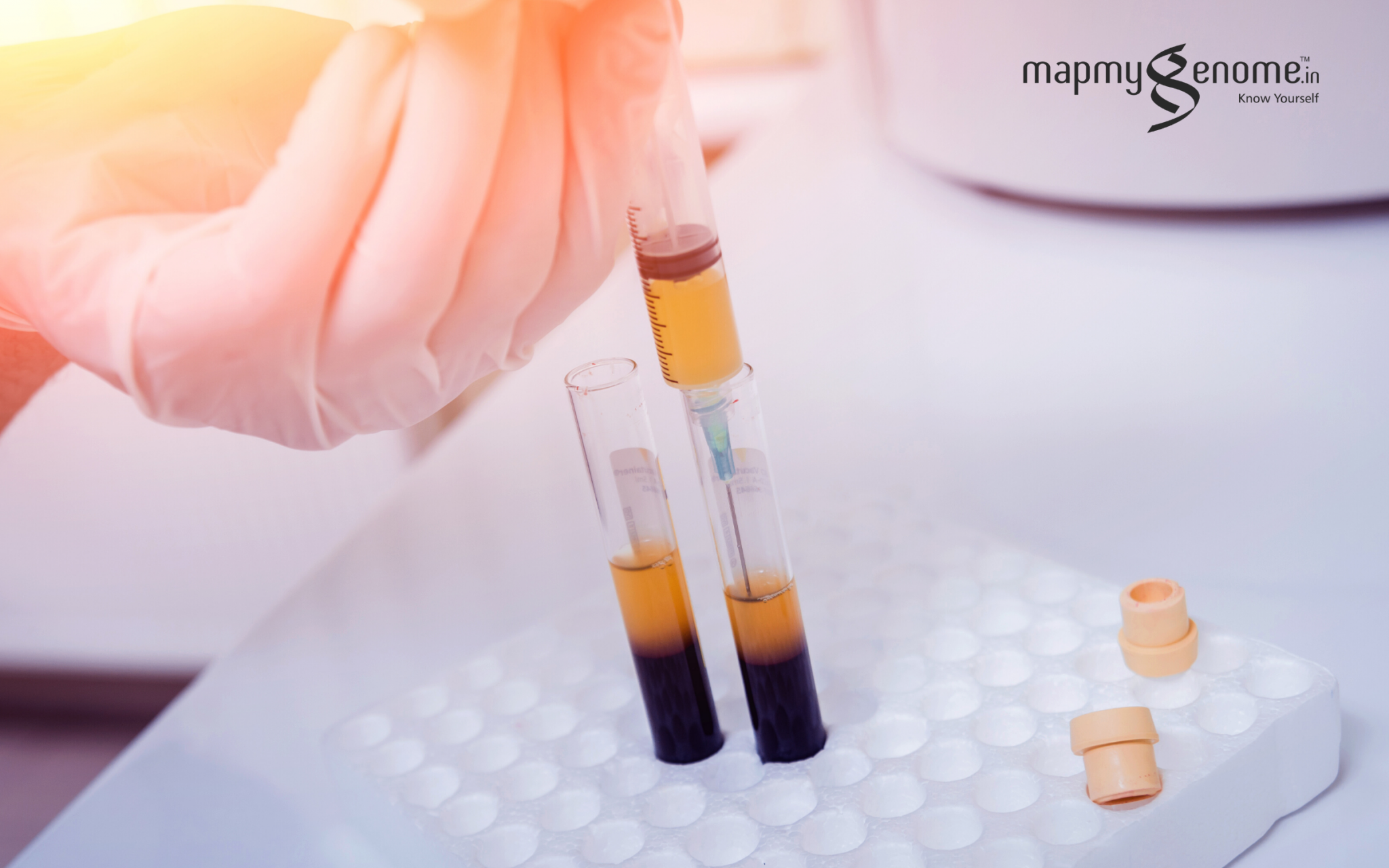
- Collection and testing: Apheresis (rather than whole blood donation) is recommended to optimize the yield of convalescent plasma. Apheresis is performed with automated technology in which whole blood is continuously centrifuged into its components (i.e. red blood cells, plasma, platelets); this allows for selective collection of the desired blood fraction with the return of the other components to the donor. Approximately 400-800 ml of plasma from a single apheresis donation provides 2-4 units of convalescent plasma for transfusion. The units are frozen within 24 hours of collection and quarantined.
- Optimal dosing and transfusion: The duration of antibody efficacy is unknown but is postulated to last between a few weeks to months. The selected dosing was based on experience with previous use of convalescent plasma therapy in SARS, where 5 ml/kg of plasma at a titer of 1:160 was utilized. Typically, prophylactic doses (in some cases only a quarter of that of the proposed treatment dose) are used.
Potential Risks
There is a possible risk for inadvertent infections, or suppression of the natural innate immune responses in patients, or patients may develop serum sickness, allergic transfusion reactions, transfusion-associated circulatory overload, etc.
Convalescent plasma therapy for COVID-19 treatment
Recent reports stated the use of plasma therapy for the treatment of COVID-19 patients. The studies were first reported at the infectious disease department, China. Five patients (age range, 36-73 years; 2 women) were treated with convalescent plasma. Convalescent plasma was administered between 10 and 22 days after the onset of infection. All 5 patients were receiving medical ventilation at the time of treatment. Following the transfusion, the body temperature normalized, and the viral load decreased with a decrease in the disease severity.
While there were certain limitations for the study, the limited sample size and study design preclude a definitive statement about the potential effectiveness of this treatment, and these observations require evaluation in clinical trials. It was not clear if the patients had improved conditions because of plasma therapy, as the patients were treated with multiple agents, antiviral drugs, etc.
The risks of COVID-19 infection are profound. Since there is substantial evidence of clinical benefits of plasma therapy in past viral infections, there is a strong precedent for such an approach in COVID-19 treatment. However, it is critically important to perform well-controlled clinical trials to confirm efficacy, thereby informing rational evidence-based decision-making.